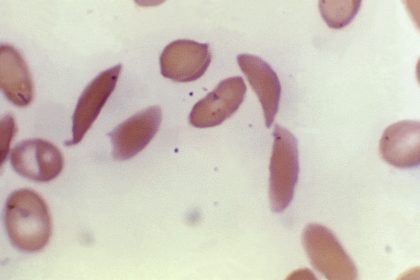
The World’s First Gene Therapy for Sickle Cell and Thalassemia Has Been Approved
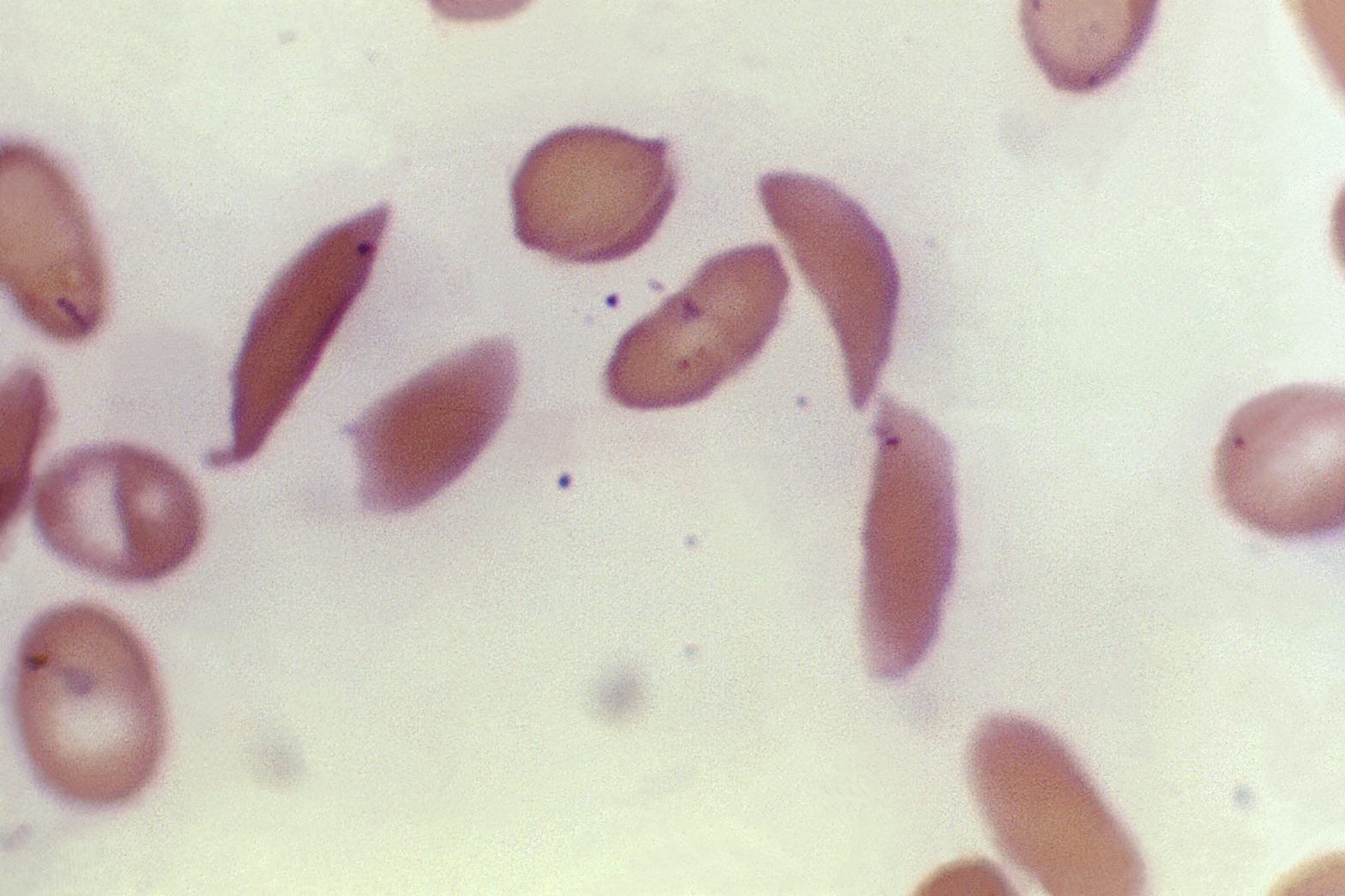
LONDON (AP) — Britain’s medicines regulator has authorized the world’s first gene therapy treatment for sickle cell disease, in a move that could offer relief to thousands of people with the crippling disease in the U.K.
In a statement on Thursday, the Medicines and Healthcare Regulatory Agency said it approved Casgevy, the first medicine licensed using the gene editing tool CRISPR, which won its makers a Nobel prize in 2020.
The agency approved the treatment for patients with sickle cell disease and thalassemia who are 12 years old and over. Casgevy is made by Vertex Pharmaceuticals (Europe) Ltd. and CRISPR Therapeutics. To date, bone marrow transplants, extremely arduous procedures that come with very unpleasant side effects, have been the only long-lasting treatment.
“The future of life-changing cures resides in CRISPR based (gene-editing) technology,” said Dr. Helen O’Neill of University College London.
“The use of the word ‘cure’ in relation to sickle cell disease or thalassemia has, up until now, been incompatible,” she said in a statement, calling the MHRA’s approval of gene therapy “a positive moment in history.”
Both sickle cell disease and thalassemia are caused by mistakes in the genes that carry hemoglobin, the protein in red blood cells that carry oxygen.
In people with sickle cell — which is particularly common in people with African or Caribbean backgrounds — a genetic mutation causes the cells to become crescent-shaped, which can block blood flow and cause excruciating pain, organ damage, stroke and other problems.
In people with thalassemia, the genetic mutation can cause severe anemia. Patients typically require blood transfusions every few weeks, and injections and medicines for their entire life. Thalassemia predominantly affects people of South Asian, Southeast Asian and Middle Eastern heritage.
The new medicine, Casgevy, works by targeting the problematic gene in a patient’s bone marrow stem cells so that the body can make properly functioning hemoglobin.
Patients first receive a course of chemotherapy, before doctors take stem cells from the patient’s bone marrow and use genetic editing techniques in a laboratory to fix the gene. The cells are then infused back into the patient for a permanent treatment. Patients must be hospitalized at least twice — once for the collection of the stem cells and then to receive the altered cells.
Britain’s regulator said its decision to authorize the gene therapy for sickle cell disease was based on a study done on 29 patients, of whom 28 reported having no severe pain problems for at least one year after being treated. In the study for thalassemia, 39 out of 42 patients who got the therapy did not need a red blood cell transfusion for at least a year afterwards.
Gene therapy treatments can cost millions of dollars and experts have previously raised concerns that they could remain out of reach for the people who would benefit most.
Last year, Britain approved a gene therapy for a fatal genetic disorder that had a list price of £2.8 million ($3.5 million). England’s National Health Service negotiated a significant confidential discount to make it available to eligible patients.
Vertex Pharmaceuticals said it had not yet established a price for the treatment in Britain and was working with health authorities “to secure reimbursement and access for eligible patients as quickly as possible.”
In the U.S., Vertex has not released a potential price for the therapy, but a report by the nonprofit Institute for Clinical and Economic Review said prices up to around $2 million would be cost-effective. By comparison, research earlier this year showed medical expenses for current sickle cell treatments, from birth to age 65, add up to about $1.6 million for women and $1.7 million for men.
Medicines and treatments in Britain must be recommended by a government watchdog before they are made freely available to patients in the national health care system.
Casgevy is currently being reviewed by the U.S. Food and Drug Administration; the agency is expected to make a decision early next month, before considering another sickle cell gene therapy.
Millions of people around the world, including about 100,000 in the U.S., have sickle cell disease. It occurs more often among people from places where malaria is or was common, like Africa and India, and is also more common in certain ethnic groups, such as people of African, Middle Eastern and Indian descent. Scientists believe being a carrier of the sickle cell trait helps protect against severe malaria.
__
AP Science Writer Laura Ungar in Louisville, Kentucky contributed to this report.
The Associated Press Health and Science Department receives support from the Howard Hughes Medical Institute’s Science and Educational Media Group. The AP is solely responsible for all content.