FDA Authorizes Pfizer COVID-19 Vaccine for Children 5 to 11
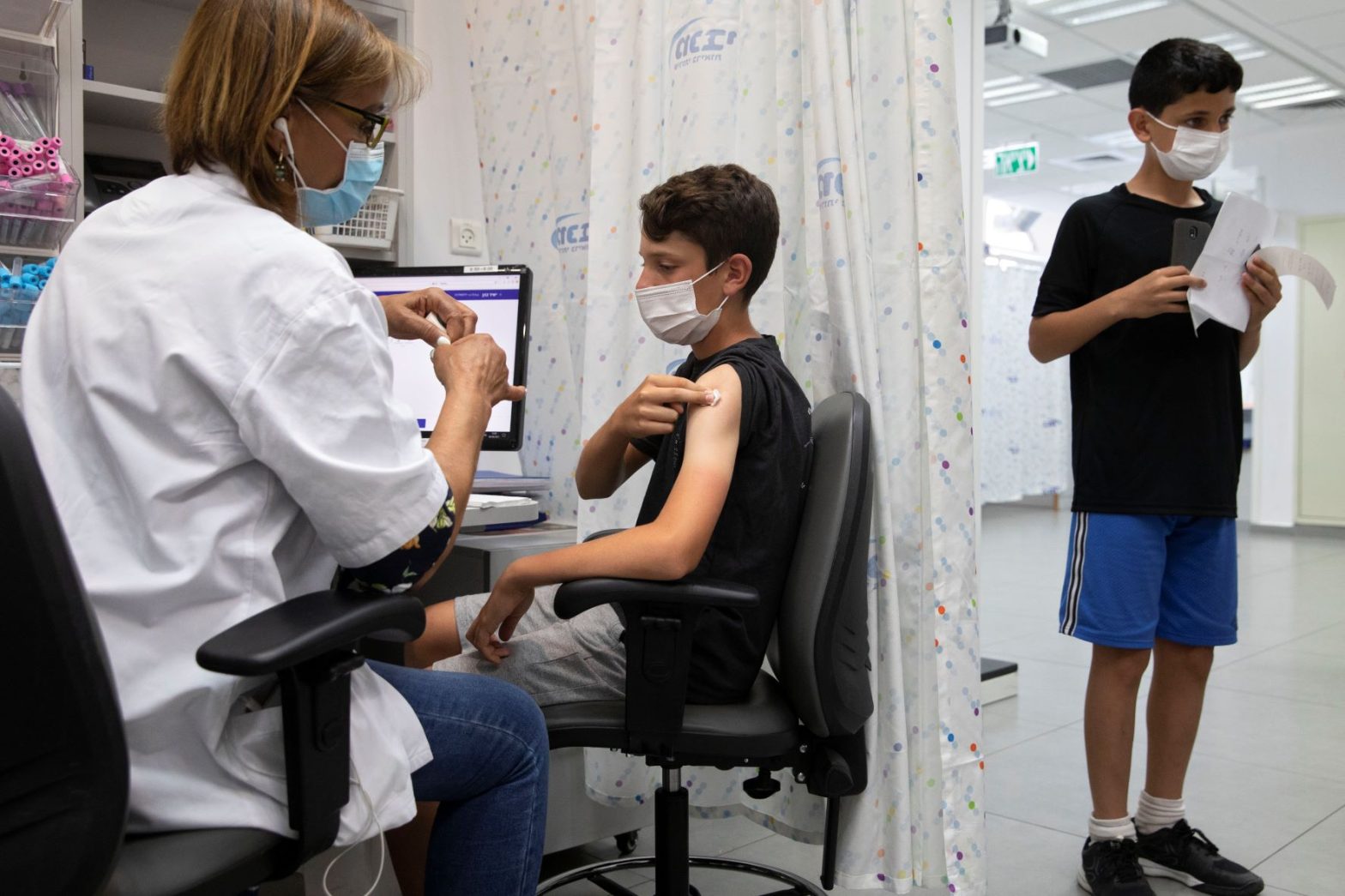
WASHINGTON — The Food and Drug Administration on Friday authorized the Pfizer-BioNTech COVID-19 vaccine for emergency use in children 5 to 11.
“As a mother and a physician, I know that parents, caregivers, school staff, and children have been waiting for today’s authorization,” said Acting FDA Commissioner Dr. Janet Woodcock in a written statement announcing the decision.
“Vaccinating younger children against COVID-19 will bring us closer to returning to a sense of normalcy,” Woodcock continued, adding, “Our comprehensive and rigorous evaluation of the data pertaining to the vaccine’s safety and effectiveness should help assure parents and guardians that this vaccine meets our high standards.”
If the Centers for Disease Control and Prevention signs off, as is expected when its Advisory Committee on Immunization Practices meets next week, children could start getting shots as early as Wednesday.
The FDA said in a press release Friday that children 5 to 11 would be administered a two-dose primary series, three weeks apart, but at a lower dose (10 micrograms) than that used for individuals 12 years of age and older (30 micrograms).
The agency went on to say that in a study involving 3,100 children aged 5 to 11, the immune response was comparable to those in earlier studies on individuals aged 16 to 25. In that study, the vaccine was deemed to be about 90.7% effective.
In addition, the FDA said no serious adverse effects have been detected in the ongoing study.
The Biden administration has promised that children’s shots will be easily accessible at pediatrician offices, community health centers, children’s hospitals and pharmacies, with 15 million doses ready to ship immediately.
States started ordering doses last week, under a formula based on how many children they have in the age group. While the school year is already well underway, the pediatric dose is arriving in time for the holidays, giving more comfort to families looking to gather older and younger people together for the first time since the early months of 2020.
According to the CDC, COVID-19 cases in children 5 to 11 comprise about 39% of cases in individuals younger than 18 years of age.
In addition, the Centers said approximately 8,300 COVID-19 cases in children 5 to 11 resulted in hospitalization.
As of Oct. 17, 691 deaths from COVID-19 have been reported in the U.S. in individuals younger than 18 years of age, with 146 deaths in the 5 to 11 age group, the CDC said.
“Today is a momentous day in the fight against the COVID-19 pandemic, with the FDA’s first emergency use authorization of a coronavirus vaccine for younger children,” House Speaker Nancy Pelosi said in a statement.
“Congress and the country are grateful to our scientists and health officials, who worked quickly and carefully to ensure that the Pfizer vaccine is safe and effective for our youngest Americans,” she said. “Getting our children vaccinated is the best way to ensure their health and safety, so they can go to school, spend time with their families and engage in our communities with the confidence of knowing they are protected from this vicious virus.
“It is heartbreaking that more than a thousand Americans lose their lives to this virus each day, nearly all of whom were unvaccinated. It is now crucial that every eligible American — especially children ages five to eleven — get vaccinated as quickly as possible, so that we can finally put an end to this pandemic,” Pelosi added.
Dan can be reached at [email protected] and at https://twitter.com/DanMcCue

























