FDA Approves Updated COVID Vaccines for New Variants
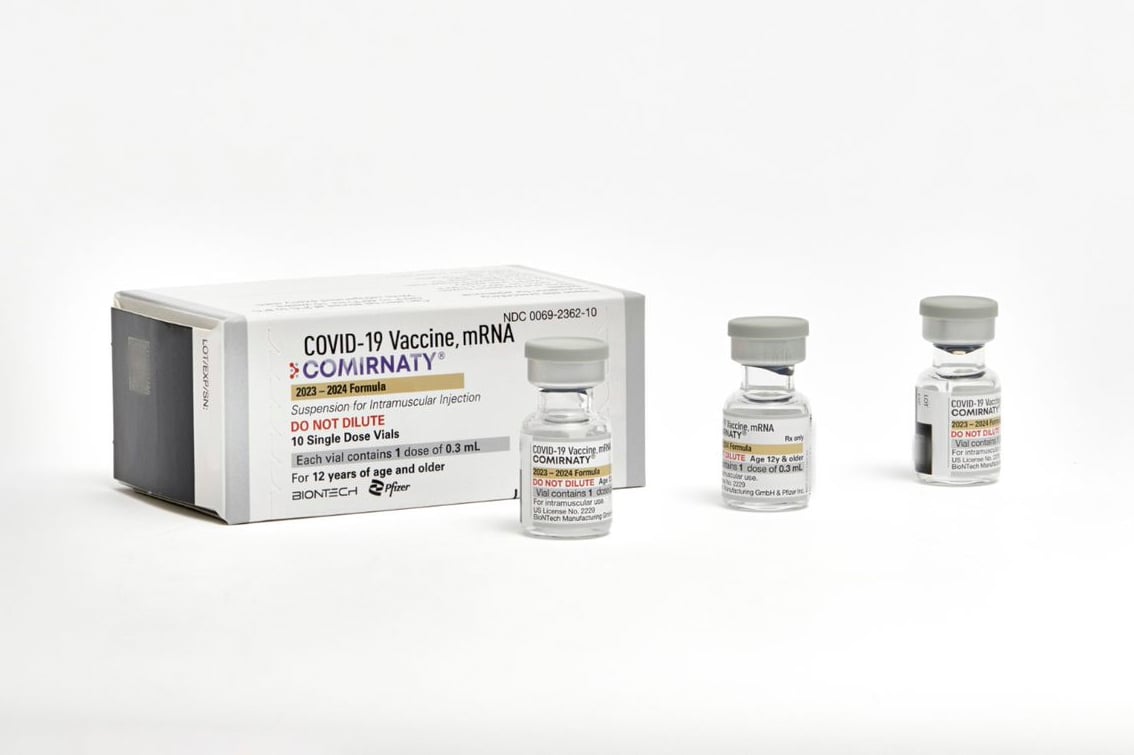
WASHINGTON — The Food and Drug Administration on Monday approved the use of updated COVID-19 vaccines to provide the public with additional protection against the virus ahead of a potential winter bump in cases.
One thing the FDA did not include in its announcement Monday afternoon was who it will recommend getting the booster shot.
That decision has been left to a group of outside advisors to the Centers for Disease Control and Prevention who are scheduled to meet on Tuesday.
Once it makes its recommendations, CDC Director Mandy Cohen is expected to promptly sign off on them.
At that point, the new shots, which are manufactured by Moderna and Pfizer-BioNTech, will be made available to the public, possibly by the end of the week.
“Vaccination remains critical to public health and continued protection against serious consequences of COVID-19, including hospitalization and death,” said Peter Marks, director of the FDA’s Center for Biologics Evaluation and Research, in a written statement.
“The public can be assured that these updated vaccines have met the agency’s rigorous scientific standards for safety, effectiveness, and manufacturing quality. We very much encourage those who are eligible to consider getting vaccinated,” he said.
The last time the CDC advisory panel recommended members of the public get COVID boosters, during last year’s omicron surge, it limited its target population to older and at-risk individuals who were already immunocompromised.
Their new recommendation could be a repeat, even in light of an increase in cases stemming from the XBB.1.5 variant that emerged earlier this summer.
The shots approved by the FDA this week were developed specifically to address that strain, but it is believed they will also be effective against the EG.5 and FL.1.5.1 variants, which descended from the summer’s variant.
The FDA is still reviewing another shot from Novavax, which differs from the mRNA vaccines made by Pfizer and Moderna in that it is based on a more traditional, protein-based platform.
Dan can be reached at [email protected] and @DanMcCue

























