NIH Evaluating Antiviral in Adults Hospitalized With COVID-19
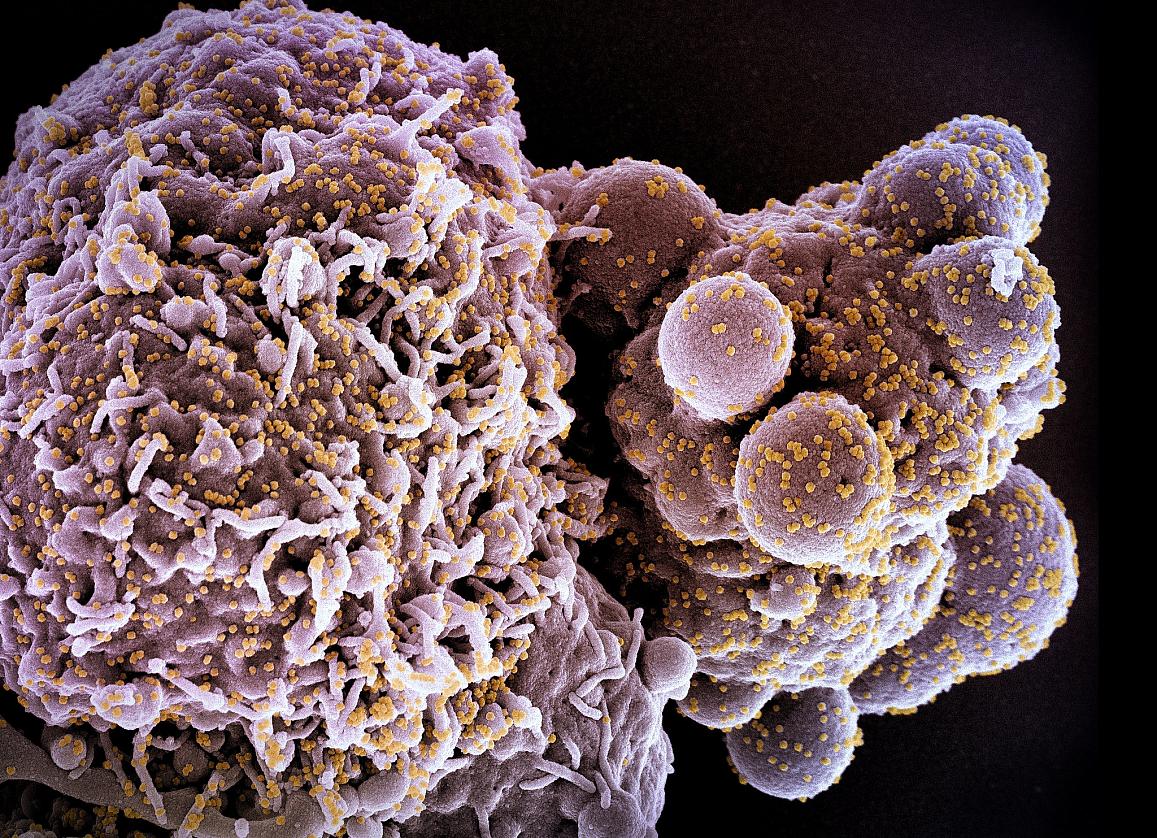
WASHINGTON — The National Institutes of Health has begun a multi-state clinical trial evaluating an “investigational antiviral” for the treatment of adults hospitalized with COVID-19.
The treatment, known as S-217622 or ensitrelvir fumaric acid, was developed in Japan by Hokkaido University and Shionogi & Co., Ltd.
The trial is assessing whether S-217622 can improve clinical outcomes for patients who are hospitalized for management of COVID-19 as compared to a placebo and will enroll approximately 1,500 people at sites worldwide.
S-217622 is the first agent to be evaluated in a new global, adaptive clinical research protocol known as Strategies and Treatments for Respiratory Infections & Viral Emergencies.
“We hope results from this trial can be applied to improve the standard of care for people with COVID-19, which still causes hundreds of deaths each day in the United States, as well as to strengthen our pandemic preparedness,” said Dr. H. Clifford Lane, deputy director for clinical research at the National Institute of Allergy and Infectious Diseases, in a written statement.
“The STRIVE protocol and clinical research infrastructure can be adapted to evaluate additional agents for COVID-19, as well as therapeutics for other respiratory pathogens,” he said.
The National Institute of Allergy and Infectious Diseases, part of NIH, is funding the trial and working in partnership with NIH’s National Center for Advancing Translational Sciences.
Developed under the auspices of the NIH Accelerating COVID-19 Therapeutic Interventions and Vaccines public-private partnership, STRIVE may be adapted to rapidly assess multiple therapeutic interventions during outbreaks of respiratory diseases, such as COVID-19 or influenza.
Findings from the ACTIV-3 trials, which evaluated therapeutics for adults hospitalized with COVID-19, indicated that ongoing viral replication may play a role in driving disease progression to critical illness.
The research team hypothesized that a significant number of patients hospitalized with COVID-19 may still benefit from antiviral therapy.
S-217622 suppresses SARS-CoV-2 replication by inhibiting the function of a key virus protein known as 3CL protease.
The antiviral reduced COVID-19 symptoms in people with mild-to-moderate COVID-19, regardless of risk factors or vaccination status, according to results shared by Shionogi from a phase 3 clinical trial conducted mainly in Japan. S-217622 was well-tolerated, and there were no treatment-related serious adverse events or deaths in the study. In November 2022 the drug received emergency regulatory approval in Japan, where it is referred to by the brand name Xocova.
The ongoing ACTIV-2 SCORPIO-HR clinical trial, also funded by NIAID, is evaluating the antiviral in the outpatient population.
Going forward, investigators will monitor and record participants’ health status every day during the first week and then on prespecified days over a 60-day period. Participants also will be asked to provide blood samples and nose swabs for laboratory tests.
The study team will conduct analyses to understand any statistically significant differences — meaning those unlikely due to chance — between the treatment group and the placebo group. The key metric they will focus on is whether people receiving S-217622 recover (defined as returning home and remaining alive through day 60) more quickly than those in the placebo group.
Participant safety will be monitored closely throughout the trial. An NIAID Medical Monitor will review any potential safety issues in real time, and an independent data and safety monitoring board will review safety and efficacy data regularly.
The pace of enrollment and, ultimately, the amount of time it takes to complete the trial will depend on the trajectory of COVID-19 cases in various parts of the world. For more information about the trial, please visit clinicaltrials.gov and search identifier NCT05605093.
STRIVE is part of the NIH ACTIV public-private-partnership, which was initiated in April 2020 to develop a coordinated research strategy for prioritizing and speeding development of the most promising treatments and vaccines for COVID-19.
STRIVE builds on the efforts of prior ACTIV master protocols that evaluated various therapeutics for people hospitalized with COVID-19. The STRIVE Scientific Committee, chaired by Professor Jens Lundgren, M.D., from the University of Copenhagen, is composed of clinical trial experts from several medical specialties including infectious diseases, pulmonology, intensive care and emergency medicine. STRIVE is also overseen by a leadership committee and a community advisory board.
The Statistical and Data Management Center at University of Minnesota, led by James Neaton, Ph.D., is managing STRIVE in collaboration with eight international coordinating centers, bringing together former sites from the ACTIV-1, ACTIV-3 and ACTIV-5 clinical trials. In all, STRIVE can enroll participants at more than 200 sites on all six inhabited continents.
NIAID conducts and supports research — at NIH, throughout the United States, and worldwide — to study the causes of infectious and immune-mediated diseases, and to develop better means of preventing, diagnosing and treating these illnesses. News releases, fact sheets and other NIAID-related materials are available on the NIAID website.
Dan can be reached at [email protected] and at https://twitter.com/DanMcCue

























