Pfizer Says COVID-19 Vaccine Appears to be 90% Effective
Stocks surged around the world Monday after Pfizer announced that early results from trials of its coronavirus vaccine suggest the shots may be 90% effective at preventing COVID-19.
But President-elect Joe Biden quickly reminded Americans that the good news does not mean it’s time to abandon face masks and social distance just yet or even in the near future.
“Even if … some Americans are vaccinated later this year, it will be many more months before there is widespread vaccination in this country,” Biden said in a statement.
Development of the vaccine does not change the “urgent reality” that Americans will have to rely on masking, distancing, and other mitigation in the months ahead, he added.
Markedly less cautious was President Donald Trump who extolled the announcement on Twitter in all caps: “STOCK MARKET UP BIG, VACCINE COMING SOON. REPORT 90% EFFECTIVE. SUCH GREAT NEWS!”
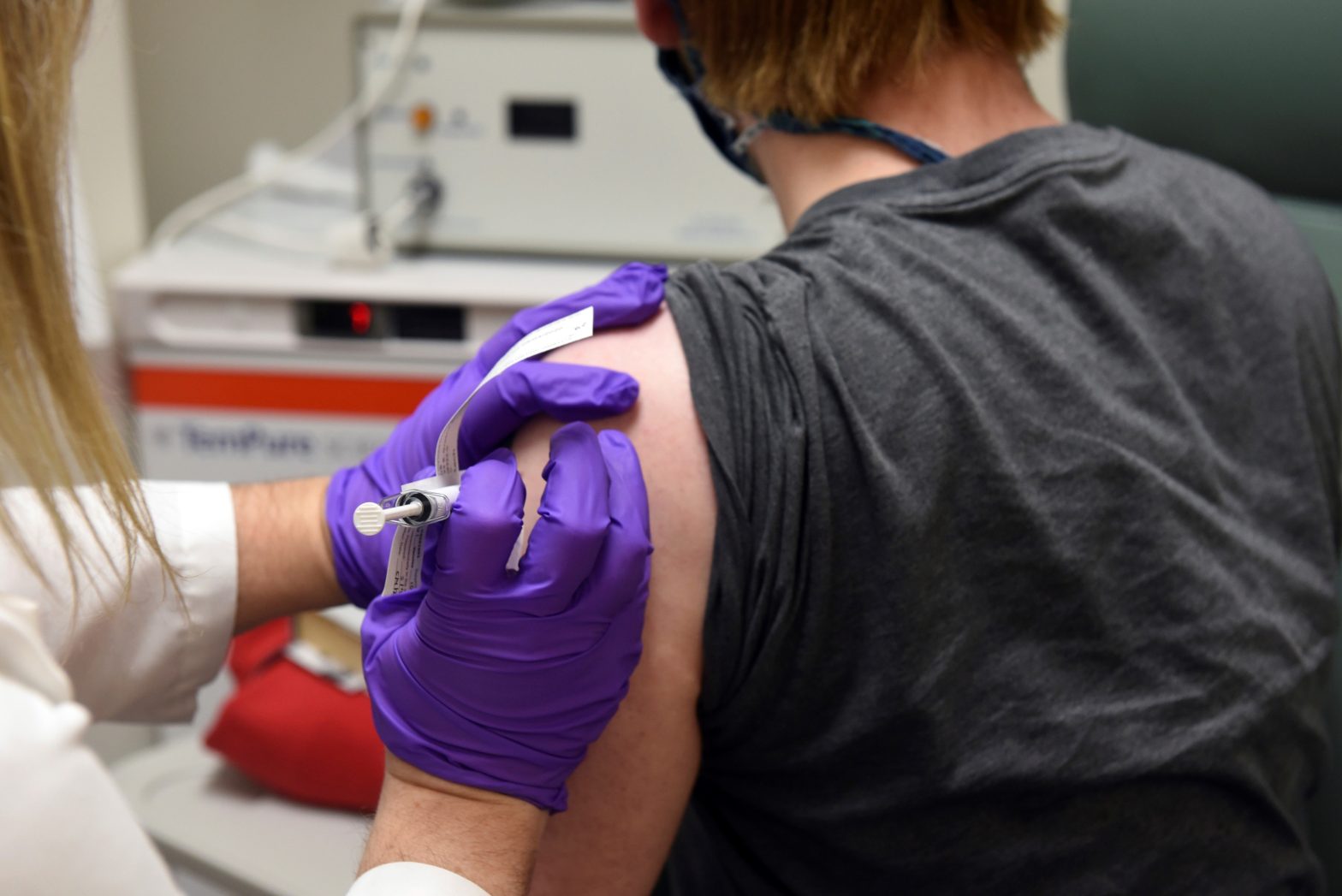
Pfizer said Monday that early results from its coronavirus vaccine suggest the shots may be a surprisingly robust 90% effective at preventing COVID-19, putting the company on track to apply later this month for emergency-use approval from the Food and Drug Administration.
Dr. Anthony Fauci, the U.S. government’s top-infectious disease expert, said the results suggesting 90% effectiveness are “just extraordinary,” adding: “Not very many people expected it would be as high as that.”
“It’s going to have a major impact on everything we do with respect to COVID,” Fauci told the Associated Press as Pfizer appeared to take the lead in the all-out global race by pharmaceutical companies and various countries to develop a well-tested vaccine against the virus.
The shots, made by Pfizer and its German partner BioNTech, are among 10 possible vaccine candidates in late-stage testing around the world — four of them so far in huge studies in the U.S.
Another U.S. company, Moderna Inc., also hopes to file an application with the FDA this month.
Still, Monday’s announcement doesn’t mean for certain that a vaccine is imminent: This interim analysis, from an independent data monitoring board, looked at 94 infections recorded so far in a study that has enrolled nearly 44,000 people in the U.S. and five other countries.
Some participants got the vaccine, while others got dummy shots.
Among the questions still to be answered are how long the vaccine’s effects last and whether it protects older people as well as younger ones.
Also, whenever a vaccine does arrive, initial supplies will be scarce and rationed, with priority likely to be given to health care workers and others on the front lines.
Pfizer has estimated that 50 million doses of its two-shot vaccine could be available globally by the end of 2020, which could cover 25 million people.
Of course, the timing of Pfizer’s announcement is likely to feed unsubstantiated suspicions from Trump supporters that the pharmaceutical industry was withholding the news until after the election.
Donald Trump Jr. tweeted: “The timing of this is pretty amazing. Nothing nefarious about the timing of this at all right?”
Pfizer Chairman and CEO Albert Bourla said on CNBC that the election was always an artificial deadline and that the data was going to be ready when it was ready. The independent data monitors met on Sunday, analyzing the COVID-19 test results so far and notifying Pfizer.
“I am very happy,” Bourla said, “but at the same time, sometimes I have tears in my eyes when I realize that this is the end of nine months, day-and-night work of so many people and how many people, billions, invested hopes on this.”
Pfizer opted not to join the Trump administration’s Operation Warp Speed, which helped a half-dozen drugmakers accelerate their vaccine testing and helped fund the work. Instead, Pfizer funded all its testing and manufacturing costs itself. The company said it has invested billions of dollars.
Pfizer doesn’t plan to stop its study until it records 164 infections among all the volunteers, a number that the FDA has agreed is enough to tell how well the vaccine is working. The agency has made clear that any vaccine must be at least 50% effective.
Yet, it was hard not to feel good about what House Speaker Nancy Pelosi called “welcome good news” on the pandemic front.
If the early evidence of the vaccine’s “extraordinary efficacy and safety holds true,” she said, Monday’s announcement could signal “a giant leap forward in our fight against the coronavirus.
Still, Pelosi said, no vaccine should be approved or distributed “one day sooner or one day later than it is ready.”
“The arrival of this vaccine is a tribute to the tireless work of scientists and the immense value of international collaboration. This vaccine is needed not only for America, but for the whole world. None of us is safe until all of us are safe from this terrible virus,” she said.
“Until an approved vaccine is widely available, however, we must still work to crush the virus with the testing, tracing, treatment, mask wearing and social distancing that are essential to preventing tens of thousands of needless deaths in the coming months,” she concluded.
























