Pfizer Asks FDA to Authorize COVID Booster Shots for Omicron BA.5
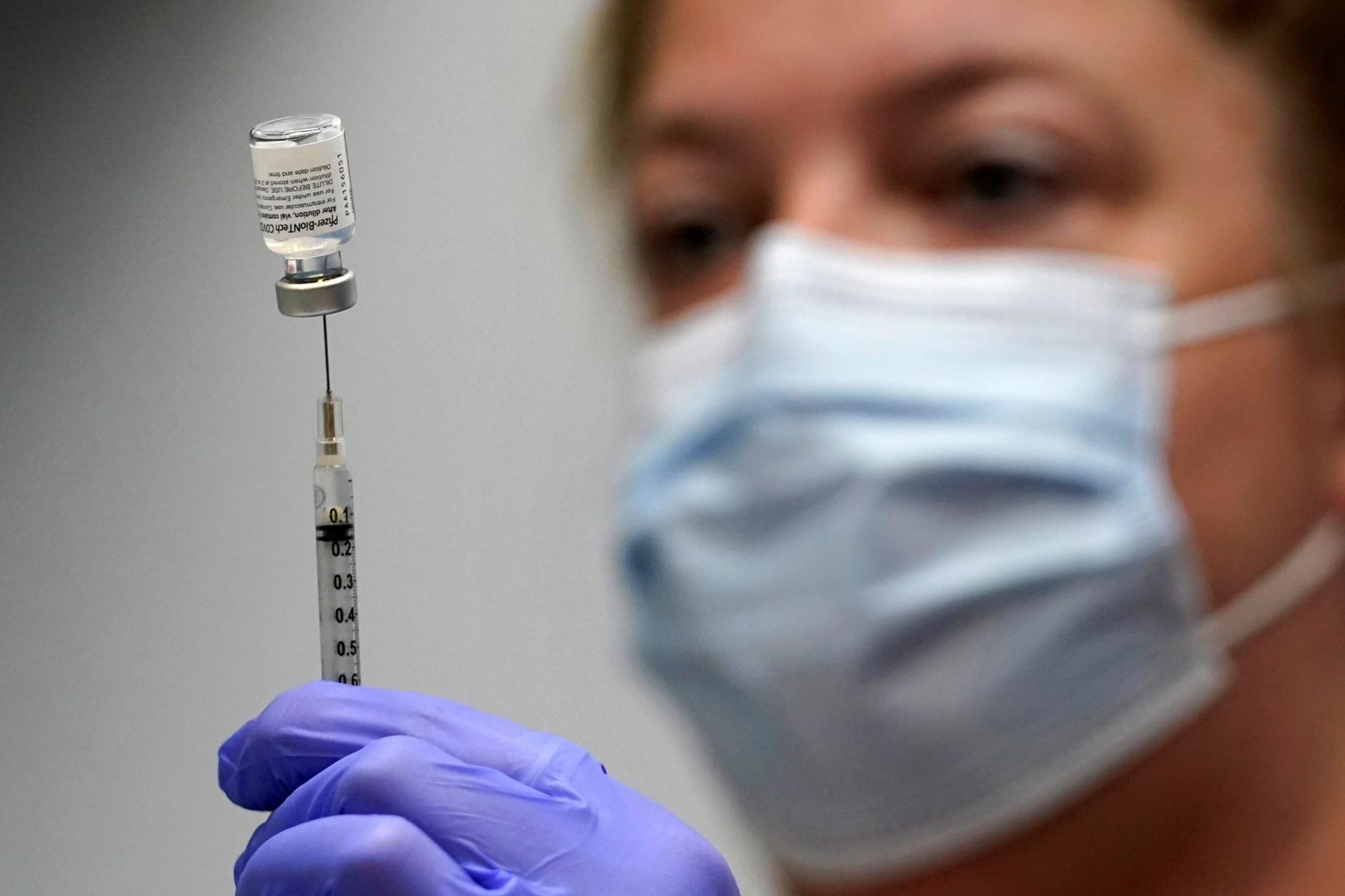
WASHINGTON — Pfizer and its German partner, BioNTech, asked the Food and Drug Administration Monday to authorize COVID booster shots that target the omicron BA.4 and BA.5 subvariants for people aged 12 and older.
The request came less than a week after Dr. Anthony Fauci, director of the National Institute of Allergy and Infectious Diseases, predicted a fall coronavirus surge is likely as cases in the U.S. start creeping up due to the highly transmissible BA.2 variant.
“I would think that we should expect that we are going to see some increase in cases as you get to the colder weather in the fall,” said Fauci, who is also chief medical advisor to President Joe Biden.
His comments were not unexpected. Other public health officials have also said they expect another mild wave of infection this fall and immunity from older shots wears off and people begin to gather more indoors as the weather grows colder.
The updated Pfizer/BioNTech vaccines would target both the original strain of the virus that first emerged in Wuhan, China, in 2019 and omicron.
“The agility of the mRNA platform, together with extensive clinical experience with the Pfizer/BioNTech COVID-19 Vaccine, has allowed us to develop, test and manufacture updated, high-quality vaccines that align to circulating strains with unprecedented speed,” said Albert Bourla, Pfizer’s chairman and chief executive officer in a written statement.
“Having rapidly scaled up production, we are positioned to immediately begin distribution of the bivalent omicron BA.4/BA.5 boosters, if authorized, to help protect individuals and families as we prepare for potential fall and winter surges,” Bourla said.
A spokesperson for the FDA said the agency will work “expeditiously” to review the Pfizer/BioNTech request “and any other submissions” it receives “in order to make modified COVID-19 vaccines available for booster vaccination in this timeframe.”
In June, Pfizer presented data to the FDA’s independent vaccine advisory committee that showed the bivalent omicron shots increased antibodies in studies involving mice at about 2.6 times the rate compared with the original vaccine.
Omicron BA.5 is now the dominant strain of COVID in the United States, making up about 90% of new infections, according to the Centers for Disease Control and Prevention.
The omicron BA.4 and BA.4.6 subvariants represent a little more than 10% of new infections taken together. These versions of omicron are more contagious than past variants of COVID.
“Given the ongoing evolution of SARS-CoV-2 and its variants, it’s of great importance that vaccines can be rapidly adapted to the major circulating omicron lineages,” said Dr. Ugur Sahin, CEO and co-founder of BioNTech, in a written statement.
“In less than three months after the FDA provided its guidance for adapted vaccines in the U.S., we are ready to ship the first doses of our omicron BA.4/BA.5-adapted bivalent vaccine, pending regulatory authorization, to provide people in the U.S. with the possibility to get a booster adapted to the currently most dominant strain of the virus,” Sahin concluded.
But Dr. Paul Offit, a member of the FDA advisory committee, told The Well News on Tuesday that he’s, at best, skeptical of the data the pharmaceutical companies have presented and wants to see more information, based on human tests.
“As a famous vaccinologist at the Wistar Institute says: ‘Mice lie and monkeys exaggerate,’” he said, referring to the independent, nonprofit research institution in Philadelphia.
“You can’t use mice to predict events in people. You just can’t,” Offit said. “I mean, let me put it this way, if mouse studies were predictive of outcomes in people, we would have had an AIDS vaccine 30 years ago.
“The bottom line is, mice aren’t people. They have a different genome … and while they help sort of point you in the right direction … I just don’t think tests on mice can be used as a proof that something is going to be valuable that you’re about to give to millions of people. I just don’t think that’s adequate. And I think that the American public deserves more data than what we’ve been given,” he said.
Offit, who by day us an infectious disease and vaccine expert at Children’s Hospital of Philadelphia, said both Pfizer and Moderna appeared before the FDA Vaccine Advisory Committee in June and made presentations on their five valent vaccines.
“What they did was, they did the study the right way,” he said. “They took people who had been inoculated with three doses of the current vaccine, and gave them either a fourth dose of the current vaccine or a fourth dose of the valent vaccine.”
The valent vaccine contained both the original recipe of the COVD vaccine, plus, for the sake of the case study, the additional omicron vaccine. At the time the two companies were trying to address BA.1, the omicron variant that is now largely gone from the U.S.
“What they found through that testing was that people who got the valiant vaccine had a higher level of neutralizing antibodies against omicron, than those who got boosted with just the ancestral or original recipe strain of the vaccine. But the amount higher was not terribly convincing. It was unlikely to be clinically significant. So some of us on the FDA vaccine advisory committee voted no,” Offit said
“So now there is an interest in having a bivalent not with the ancestral strain on the vaccine plus omicron, but of the ancestral stain and BA.4/BA.5, which are two strains that are very similar.
“But now, they don’t have data in people,” he said. “And I think it is important that they show there’s a dramatically higher level of neutralizing antibodies against BA.4/BA.5 when you give the the bivalent vaccine to actual people, as compared to just giving them the ancestral strain of the vaccine.”
Asked how long the additional testing would take, Offit suggested it could probably be done in just a month’s time.
“Now what you’d really like is not only antibody data, but also what’s called T-cell data, cellular immunity data; you’d really like both of those, but at least give us the neutralizing antibody data.
“I mean, you’re not going to get efficacy studies. You’re not going to get studies to show that it works to protect against disease. But at the very least do immunogenicity studies that look at the immune response, which is predictive of protection. But mice [studies], no,” the doctor said.
Dan can be reached at [email protected] and at https://twitter.com/DanMcCue.
























