FDA Clears Single-Shot Vaccine for Use
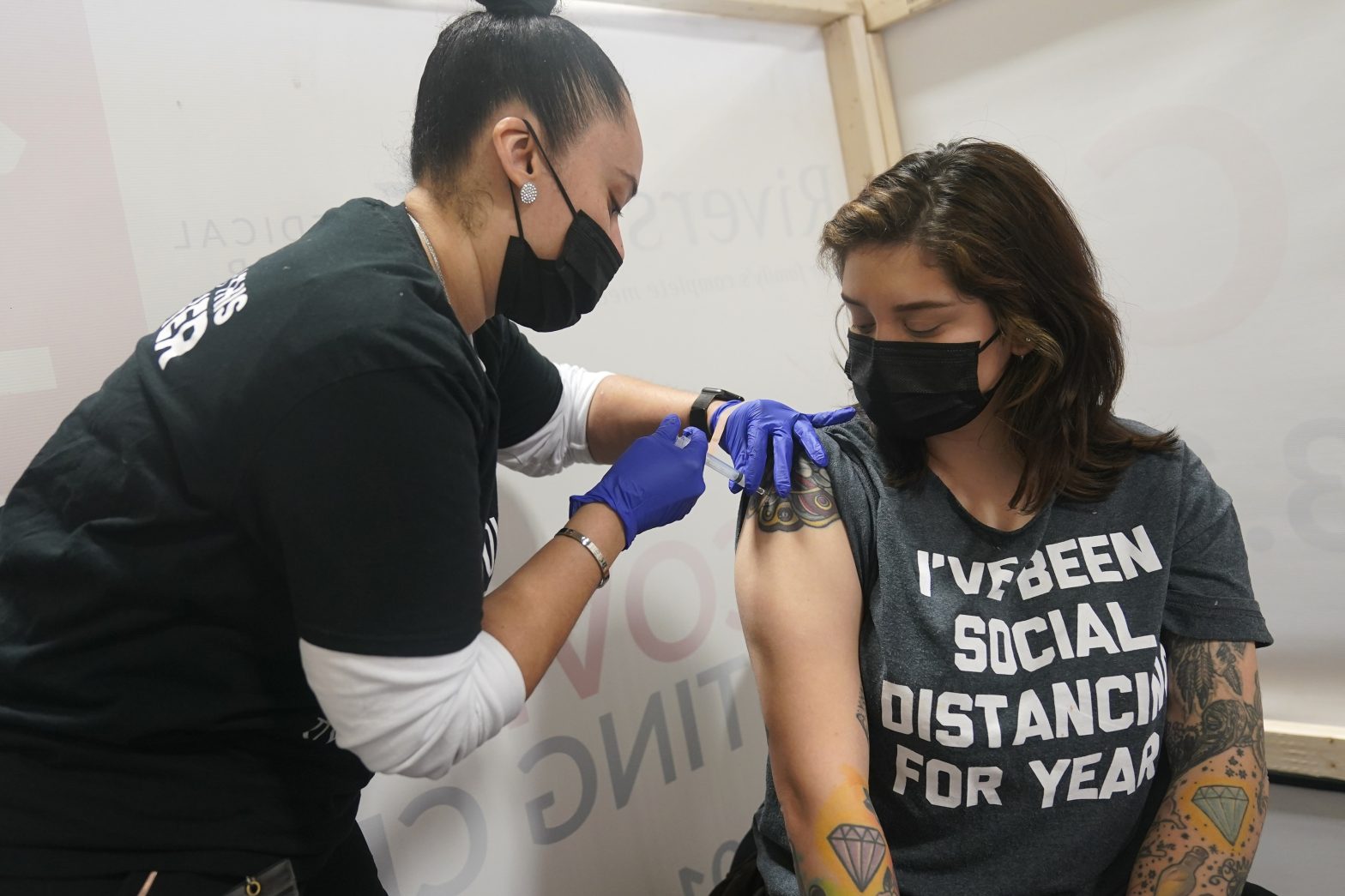
WASHINGTON – On Saturday, the Food and Drug Administration gave emergency authorization to a third coronavirus vaccine. This vaccine, unlike the Pfizer and Moderna vaccines which received emergency authorizations in December, is a single shot vaccine.
Placebo-controlled, randomized studies of 43,783 participants across eight countries, including the U.S., proved that the Janssen vaccine is safe enough to receive the green light and causes only mild to moderate side effects, the FDA said. The agency’s written statement on the vaccine reported that it is 77% effective in preventing severe/critical coronavirus cases that happen at least two weeks after vaccination and 85% effective in preventing severe/critical cases at least 28 days after vaccination.
Johnson & Johnson, the parent company of Janssen Biotech, has announced its intention to ship the vaccines using its established cold chain supply transport channels.
The “emergency use authorization” means that Janssen Biotech, Inc.’s single-dose vaccine can be distributed to adults age eighteen and older in the U.S. Under emergency use, the FDA can temporarily allow unapproved medical products, in the absence of “adequate, approved, and available alternatives,” if it is determined that there is a public health emergency that could possibly hurt national security. The Secretary of Health and Human Services declared coronavirus just such an emergency in February of 2020.
Peter Marks, director of the FDA’s Center for Biologics Evaluation and Research, commented that Janssen’s vaccine adds to “the medical toolbox” in the fight against coronavirus.
“The authorization of this vaccine expands the availability of vaccines, the best medical prevention method for COVID-19, to help us in the fight against this pandemic, which has claimed over half a million lives in the United States,” Acting FDA Commissioner Janet Woodcock said in a written comment.