Supreme Court to Review Abortion Medication Dispute
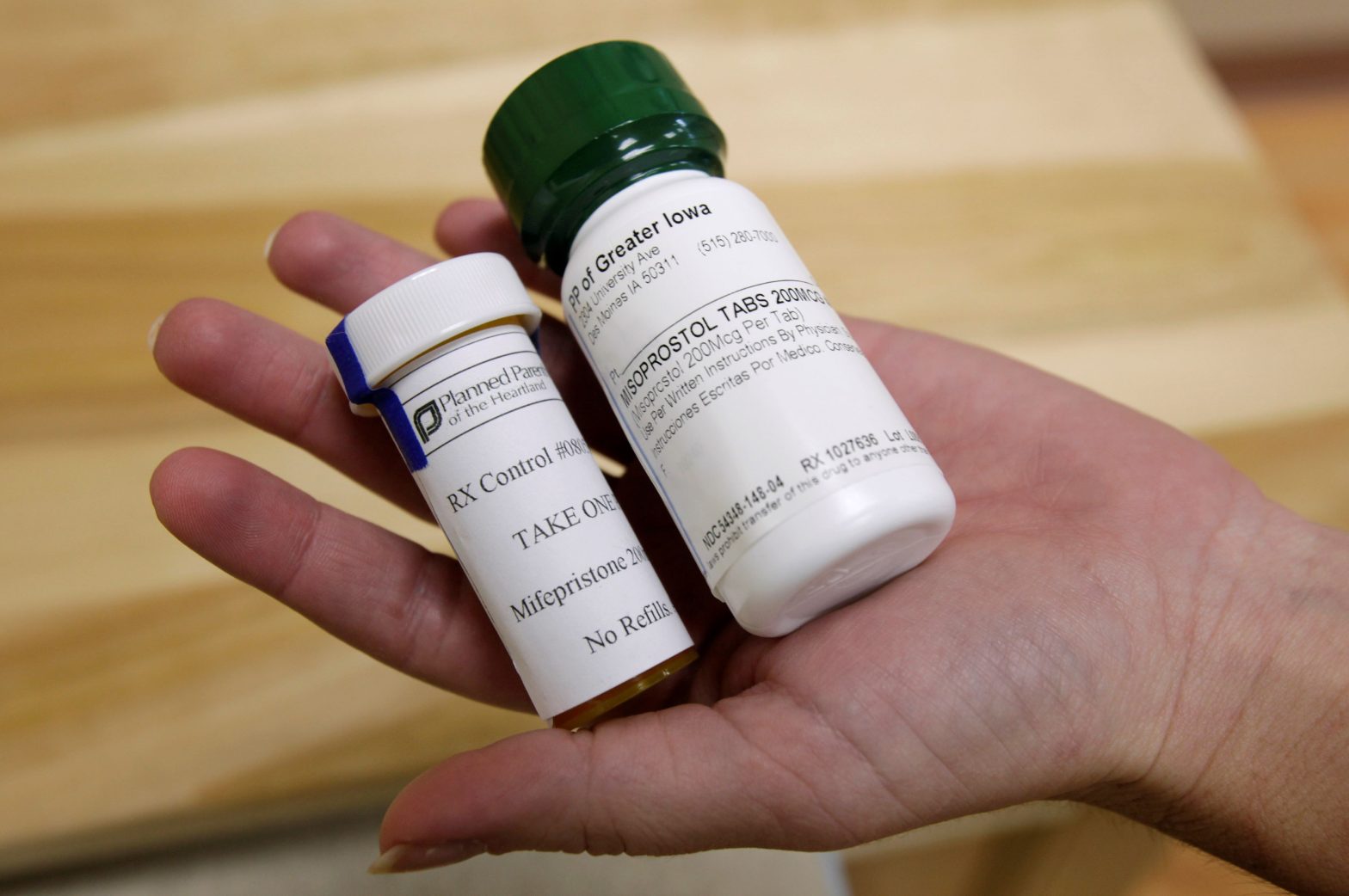
WASHINGTON — The Supreme Court announced Wednesday that it will review a dispute over the popular abortion medication mifepristone, a move that could have a sweeping impact not only on its use going forward, but also on the regulatory authority of the Food and Drug Administration.
As is their custom, the justices did not explain their rationale for taking up the case.
But a year after the court’s landmark decision in Dobbs v. Jackson Women’s Health Organization, in which they said regulatory authority over abortion belongs to the states, the justices are once again willingly thrusting themselves into a case involving abortion access.
At issue is access to mifepristone, a safe and effective medication abortion drug that was approved by the FDA in 2000 and is used in more than half of all abortions in the United States.
In April 2023, U.S. District Judge Matthew Kacsmaryk issued a preliminary injunction in a case filed in Amarillo, Texas, suspending the agency’s approval of mifepristone.
In doing so, Kacsmaryk wrote, “The court does not second-guess FDA’s decision-making lightly, but here, FDA acquiesced on its legitimate safety concerns — in violation of its statutory duty — based on plainly unsound reasoning and studies that did not support its conclusions.”
Elsewhere in his ruling, Kacsmaryk said the FDA overstepped its authority in approving mifepristone, in part, by using a specialized review process reserved for drugs to treat “serious or life-threatening illnesses.”
In doing so, the judge effectively ignored FDA arguments that its own regulations make clear that pregnancy is a medical condition that can sometimes be serious and life-threatening, instead calling it a “natural process essential to perpetuating human life.”
In October, the 5th U.S. Circuit Court of Appeals largely upheld Kacsmaryk’s injunction. Though it allowed mifepristone’s approval to stand, it put on hold subsequent changes the FDA had made to rules governing its distribution, including one that made the drug available to women by mail.
In the meantime, U.S. District Judge Thomas Rice, in Washington state, issued a separate injunction, forcing the FDA to maintain the distribution of mifepristone in 16 states and the District of Columbia regardless of the Texas or 5th Circuit rulings.
The justices did not announce a date for oral argument in the consolidated cases it will hear: F.D.A. v. Alliance for Hippocratic Medicine, and Danco Laboratories v. Alliance for Hippocratic Medicine.
At least until it rules, likely in late June, the FDA’s approval of mifepristone – and its current level of availability – will remain undisturbed.
In a statement issued by the White House, Press Secretary Karine Jean Pierre said the 5th Circuit’s decision in October “threatens to undermine the FDA’s scientific, independent judgment and would reimpose outdated restrictions on access to safe and effective medication abortion.”
It comes at a time, she said, when “across the country, we’ve seen unprecedented attacks on women’s freedom to make their own health decisions.
“States have imposed extreme and dangerous abortion bans that put the health of women in jeopardy and that threaten to criminalize doctors for providing the health care that their patients need and that they are trained to provide,” Jean Pierre continued. “No woman should be unable to access the health care that she needs. This should not happen in America, period.
“This administration will continue to stand by FDA’s independent approval and regulation of mifepristone as safe and effective,” she said, adding, “as the Department of Justice continues defending the FDA’s actions before the Supreme Court, President Biden and Vice President Harris remain firmly committed to defending women’s ability to access reproductive care.”
She concluded her statement by saying the White House continues to urge Congress to pass a law restoring the abortion protections in the high court’s 1971 decision in Roe v. Wade, which she called “the only way to ensure the right to choose for women in every state.”
On Capitol Hill, Sen. Patty Murray, D-Wash., who led members of Congress in filing an amicus brief in the case, said, “It is essential that the Supreme Court overturn the Fifth Circuit’s ruling and definitively reject the plaintiffs’ outrageous, politically motivated efforts to drastically restrict access to necessary and lifesaving abortion care throughout the entire country.”
“Today’s decision by the Supreme Court to hear this case means they will have the opportunity to reject the unprecedented, dangerous, and illegitimate arguments made by the plaintiffs in this case,” she continued.
“I’ve been clear all along that this case has nothing to do with the safety or efficacy of FDA-approved mifepristone — which is not up for debate — but about Republicans’ anti-women, anti-science, and anti-abortion agenda which they continue to force on the American people despite repeated and overwhelming rejection at the ballot box and in the court of public opinion.
“Beyond the truly devastating harm this case would do to women’s access to essential health care — at a time where medication abortion accounts for more than half of abortions in the U.S. — the implications for other FDA-approved medications that Americans rely on are enormous,” Murray said.
“It is essential that the Supreme Court overturn the 5th Circuit’s ruling and definitively reject the plaintiffs’ outrageous, politically motivated efforts to drastically restrict access to necessary and lifesaving abortion care throughout the entire country.”
Dan can be reached at [email protected] and at https://twitter.com/DanMcCue