Gene Editing Treatment Used in Human Subjects With Rare Genetic Blindness
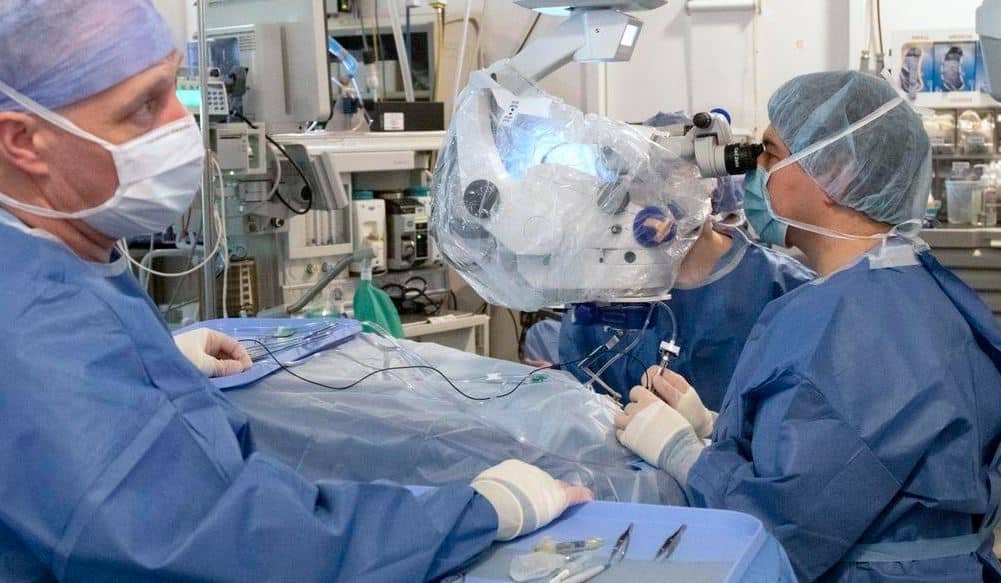
WASHINGTON — New data presented by researchers from Editas Medicine, a leading genome editing company, reveals that gene editing treatments are not only safe in humans, but may hold promise of treating a rare retinal disease that leads to blindness.
“We believe these findings validate the fundamental notion that we can safely deliver a clinically effective gene editing medicine directly to patients in order to treat their ocular disease,” said James Mullen, chairman and CEO of Editas Medicine, during the 19th International Symposium on Retinal Degeneration.
The trial is the first in the United States to use the gene editing tool called the CRISPR-Cas9 in humans to treat a condition known as CEP290-Related Retinal Degeneration, a rare retinal disease which causes progressive vision loss and blindness in children within the first decade of life.
There are currently no approved treatments for the disorder, which researchers said affects about three out of 100,000 newborns in the United States.
The genetic condition, which is inherited from two copies of a defective gene from each parent, is characterized by early loss of photoreceptors in the eye which typically remain intact until adulthood.
That’s why Editas researchers believe that early intervention of gene correction may serve as an effective solution.
“If you can repair at least one of these copies, you can potentially reverse the disease,” said Lisa Michaels, chief medical officer, Editas Medicine, during the symposium.
To conduct the trial, which takes the design of a standard dose escalation study, the researchers enrolled six participants with a clear genetic diagnosis of CEP290-Related Retinal Degeneration.
The six participants were divided into two cohorts consisting of adults 18 years and older receiving low and medium doses of the gene editing treatment which was surgically administered through an eye.
The participants were then followed every three months within the first year following the surgical procedure, and less thereafter for two more years to fully assess the safety and efficacy.
An early analysis from researchers shows that two out of the three subjects in the mid-dose cohort showed signs of efficacy, with sustained improvement in the participants’ ability to detect lower levels of light and improvement in navigation performance.
“Small improvements, such as being able to see obstacles better and see the face of a family member, or even get around in the dark, can have a real positive impact on a person’s life,” said Michaels.
When Editas researchers embarked on the study no one had previously attempted gene editing in the body, as it was not known how humans would react to the CRISPR-Cas9 enzymes, especially when delivered to the eye.
“There was a lot of concern about the potential of immune responses,” said Michaels.
“The observations we have collected to date support the safety of using CRISPR-Cas9 in this clinical setting and for the treatment of human disease. Importantly, we have not observed any serious adverse events associated with the treatment,” continued Michaels.
The next phase of the study will involve the use of gene editing treatment in adult patients at the highest planned dose.
Researchers also recently started the enrollment process for a pediatric cohort of subjects between 3-17 years-old that will receive a mid-dose treatment, as they believe there is the strongest potential benefit of this therapy for a younger population.
“We do see early signs that productive edits have occurred and potential signs of clinical benefit for the patients treated,” said Michaels.
Alexa Hornbeck can be reached at [email protected]

























