DOJ, Drug Maker Ask Supreme Court to Intervene in Abortion Pill Case
WASHINGTON — The Department of Justice and drug company Danco Laboratories asked the Supreme Court on Friday to continue to afford women access to a popular abortion drug, while the legal battle rages on concerning its use.
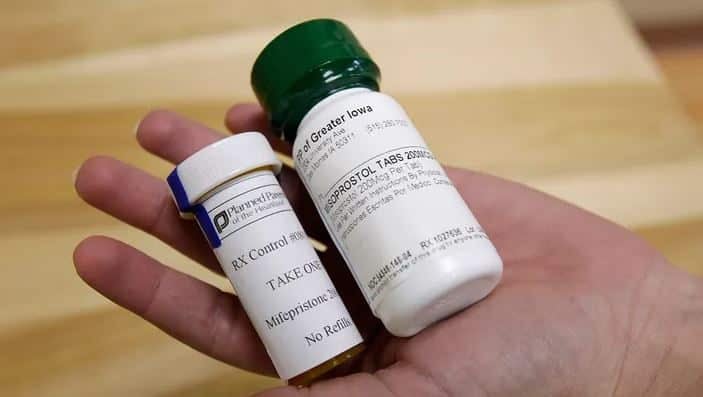
Mifepristone was approved by the Food and Drug Administration in 2000 and is used in combination with a second drug, misoprostol, to chemically terminate an unintended pregnancy in what is termed a “medication abortion.”
On April 7, a federal judge in Texas, Matthew Kacsmaryk, tossed out the FDA’s approval claiming the agency had succumbed to political pressure when it approved mifepristone initially, and continued to bow to that pressure as it gradually lifted restrictions to its use over time.
“The court does not second-guess FDA’s decision-making lightly,” Kacsmaryk wrote in the ruling. “But here, FDA acquiesced on its legitimate safety concerns — in violation of its statutory duty — based on plainly unsound reasoning and studies that did not support its conclusions.”
On Wednesday night, the 5th U.S. Circuit Court of Appeals gave the Food and Drug Administration a partial victory by declining to suspend the agency’s original approval — a move that would have immediately made the distribution of the pill unlawful.
However, the appellate panel upheld some of Kacsmaryk’s decision, including a provision that blocked the mailing or prescribing of the drug without an in-person visit to a doctor.
The 5th Circuit ruling also scaled back the FDA’s approved timeline for using mifepristone. The agency had said the drug is safe to use through 10 weeks; the appeals court demurred, and said the drug shouldn’t be used after the seventh week of pregnancy.
In the government’s filing in the Supreme Court on Friday, Solicitor General Elizabeth Prelogar argued the court should put Kacsmaryk’s entire decision on hold until all legal questions surrounding it are settled. The case was originally filed by the Alliance Defending Freedom, a conservative legal group.
“This application concerns unprecedented lower court orders countermanding FDA’s scientific judgment and unleashing regulatory chaos by suspending the existing FDA-approved conditions of use for mifepristone,” Prelogar wrote.
Separately, Danco Laboratories, which makes Mifeprex, the brand version of the pill, said it would be “irreparably harmed” if the decision goes into effect because it “will be unable to both conduct its business nationwide and comply with its legal obligations.”
In both cases, the petitioners ask the court to consider whether to quickly schedule oral arguments in the matter or issue a ruling on an expedited basis.
The request comes nearly a year after the court ruled 5-4 that its decision in Roe v. Wade was wrong and the U.S. Constitution does not confer on women a right to obtain an abortion.
It also is playing out against the backdrop of a separate ruling on mifepristone in Washington state that wholly contradicts Kacsmaryk’s decision.
On Thursday, U.S. District Judge Thomas O. Rice further clarified his ruling in that case, saying the FDA cannot do anything that would jeopardize the availability of mifepristone “irrespective” of what the Texas court and 5th Circuit said.
When mifepristone was initially approved, the FDA limited its use to up to seven weeks of pregnancy.
It also required women to make three in-person office visits: the first to administer mifepristone, the next to receive the second drug, misoprostol, and the third to address any complications.
It also required a doctor’s supervision and a reporting system for any serious consequences of the drug.
In March 2016, the FDA updated the approved label for mifepristone, a move that had several implications.
First, it reaffirmed that medication-induced abortion is very safe and highly effective.
Secondly, it dramatically reduced the barriers associated with using the drug. It did this by reducing the number of tablets of mifepristone needed, thereby reducing the cost.
At the same time, because the new label allowed women to take misoprostol at home and to follow up with their care provider over the phone, rather than in person, they now had to make fewer trips to the doctor’s office.
The updated label also allows mid-level providers — such as nurse midwives, nurse practitioners and physician assistants — to administer the drugs, which also helped increase access to the procedure.
In 2017, the year the changes went into effect, medication abortion accounted for 39% of abortions but by 2020 had increased to become the most common method, accounting for 53% of all abortions.
And reliance on medication abortion has only increased since the court overturned Roe v. Wade.
According to the FDA, more than 5.6 million women in the U.S. had used the drug as of June 2022, when the court’s decision was handed down.
Between then and now, the agency received 4,200 reports of complications in women — less than one-tenth of 1% of women who took the drug.
Dan can be reached at [email protected] and at https://twitter.com/DanMcCue
Update: Justice Samuel Alito has put lower court rulings restricting access to abortion pill mifepristone on hold until Wednesday night, April 19, and has instructed challengers to file their briefs by noon on Tuesday. See orders here: https://www.supremecourt.gov/orders/courtorders/041423zr1_2c8f.pdf; https://www.supremecourt.gov/orders/courtorders/041423zr2_c9dh.pdf























