Senate Approves $8.3 Billion Coronavirus Bill
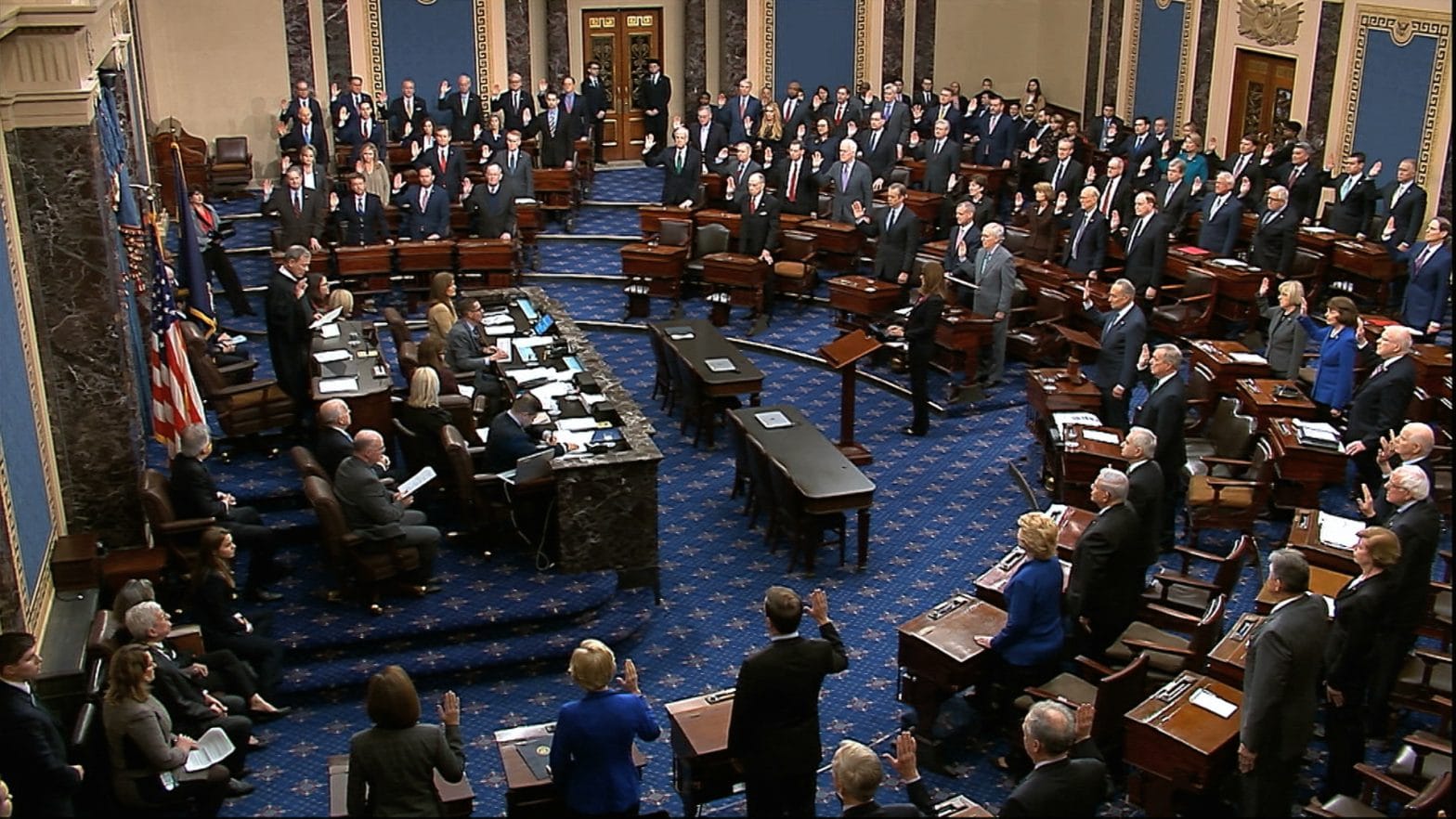
WASHINGTON – The Senate voted 96-1 on Thursday to approve $8.3 billion in funding to fight the coronavirus, sending the measure to the White House, where President Trump is expected to sign it.
The House overwhelmingly approved the same measure Wednesday with a bipartisan vote of 415-2.
The bill provides $7.76 billion to agencies combating the coronavirus. It also authorizes another $500 million in waivers for Medicare telehealth restrictions.
The measure includes $2.2 billion to help federal, state and local public health agencies prepare for and respond to the coronavirus, allocating funds for everything from lab testing to locating individuals who might have had contact with infected people.
As of Thursday afternoon, there have been 99 U.S. cases in 13 states, and 10 people have died, according to the Centers for Disease Control and Prevention.
“What we have learned from combatting similar viruses … is that time is of the essence,” said U.S. Rep. Diana DeGette, a Colorado Democrat who chairs the House Energy and Commerce Subcommittee on Oversight and Investigations.
The subcommittee oversees the nation’s health care industry.
About $435 million in the emergency supplemental package supports foreign efforts to control coronavirus. Another $300 million would respond to humanitarian needs.
Congress is approving far more than the Trump administration’s suggested $1.25 billion. Both Republicans and Democrats criticized the president for what they described as his weak response.
During a House Homeland Security Committee hearing Wednesday, Thomas Inglesby, director of the Center for Health Security at the Johns Hopkins Bloomberg School of Public Health, testified about the amount of funding needed.
“In 2009, Congress appropriated $7.7 billion for the H1N1 influenza pandemic, and in 2014, $5.4 billion was appropriated for the Ebola response,” he said in his testimony. “COVID-19 will require perhaps twice as much money as Ebola or more.”
COVID-19 is the medical term for coronavirus. U.S. health officials say it could take a year-and-half to develop a vaccine.
In China, where the virus started, the government is reporting its prevention efforts have slowed the spread of the disease. Nevertheless, it is being reported in about 100 countries.
Several House and Senate hearings have been held in recent days leading to the vote on the $8.3 billion emergency funding. Many of them included comments from lawmakers criticizing the Trump administration.
They accused President Donald Trump of downplaying the threat in a way that could help fuel the epidemic if Americans remain unprepared.
A House Foreign Affairs subcommittee held a hearing to seek answers from senior administration health officials about what they are doing to control the outbreak.
Robert Redfield, director of the Centers for Disease Control and Prevention, tried to defend the Trump administration by testifying about what he called a “multi-layered, aggressive containment” effort by U.S. health officials. It includes targeted travel restrictions, quarantines and working with the World Health Organization to combat the virus internationally.
“The risk to the American public is low,” Redfield said.
Some lawmakers asked whether the outbreak that started in China could lead to xenophobia toward persons of Asian descent, such as by refusing to have any contact with them.
Redfield said xenophobia has “no role in public health.”
He also cautioned against panic reactions, such as buying masks to wear in public. He said they were unnecessary now and could lead to a shortage.
“These masks really need to be prioritized for health care practitioners or people who have the virus and are quarantined at home,” Redfield said.
Some lawmakers were skeptical about whether the risk to Americans is small.
Rep. Ted Lieu, a California Democrat, said most coronavirus patients infect two or three persons before they are diagnosed with the disease, which has a two-week incubation period.
“There could be a whole cluster of cases,” Lieu said.