Fourth Death Attributed to Recalled Artificial Tears
ATLANTA — A fourth death has been linked to a recalled brand of artificial tears made by EzriCare and Delsam Pharma, as the number of infections with a highly drug-resistant bacteria grew to 81 across 18 states, the Centers for Disease Control and Prevention reports.
The CDC has been collaborating with the Food and Drug Administration and state and local health departments since May 2022 to investigate a multistate outbreak of an extremely drug-resistant strain of the bacteria Pseudomonas aeruginosa.
The outbreak strain, carbapenem-resistant Pseudomonas aeruginosa with Verona integron-mediated metallo-β-lactamase and Guiana extended-spectrum-β-lactamase (VIM-GES-CRPA), had never been reported in the United States prior to this outbreak.
The outbreak is associated with multiple types of infections, including eye infections, some of which have resulted in the surgical removal of the eyeball.
The investigation ultimately identified EzriCare artificial tears as a common exposure for many patients and the manufacturer, Global Pharma of Chennai, India, immediately recalled the products associated with the outbreak.
Additional cases were confirmed after the recall date, but investigators said this was due to the time it takes for testing to confirm the outbreak strain and because of the retrospective reporting of infections.
Of the seven patients who had specimens collected after the recall, most either resided in long-term care facilities with other known cases or reported use of a recalled brand of artificial tears.
Most patients reported using artificial tears. Patients reported over 10 different brands of artificial tears, and some patients used multiple brands.
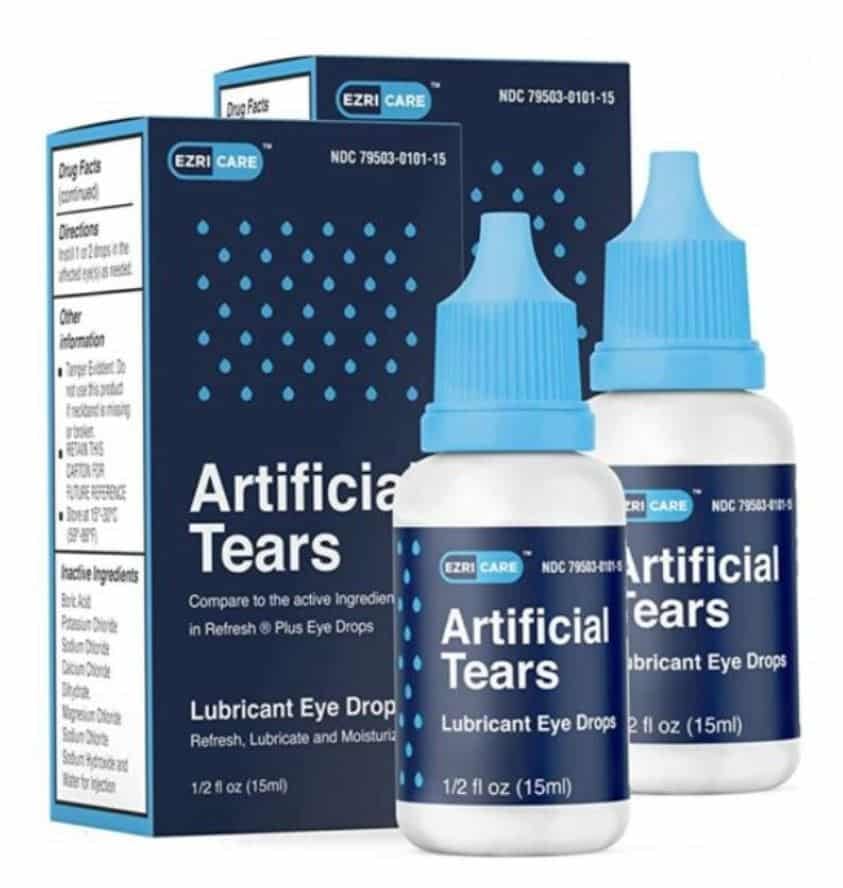
EzriCare Artificial Tears, a preservative-free, over-the-counter product packaged in multidose bottles, was the brand most commonly reported. This was the only common artificial tears product identified across the four health care facility clusters.
Laboratory testing by the CDC identified the presence of VIM-GES-CRPA in opened EzriCare bottles from multiple lots; these bottles were collected from patients with and without eye infections and from two states.
According to the CDC, VIM-GES-CRPA recovered from opened products match the outbreak strain.
Testing of unopened bottles of EzriCare Artificial Tears by the FDA also identified bacterial contamination.
To date, Global Pharma has voluntarily recalled three products associated with the outbreak: EzriCare Artificial Tears, Delsam Pharma Artificial Tears, and Delsam Pharma Artificial Ointment.
The CDC and FDA recommend clinicians and patients stop using and discard EzriCare Artificial Tears and two additional products made by the same manufacturer, Delsam Pharma’s Artificial Tears, and Delsam Pharma’s Artificial Ointment.
Dan can be reached at [email protected] and @DanMcCue