FDA Revokes Emergency Use of Hydroxychloroquine to Fight Coronavirus
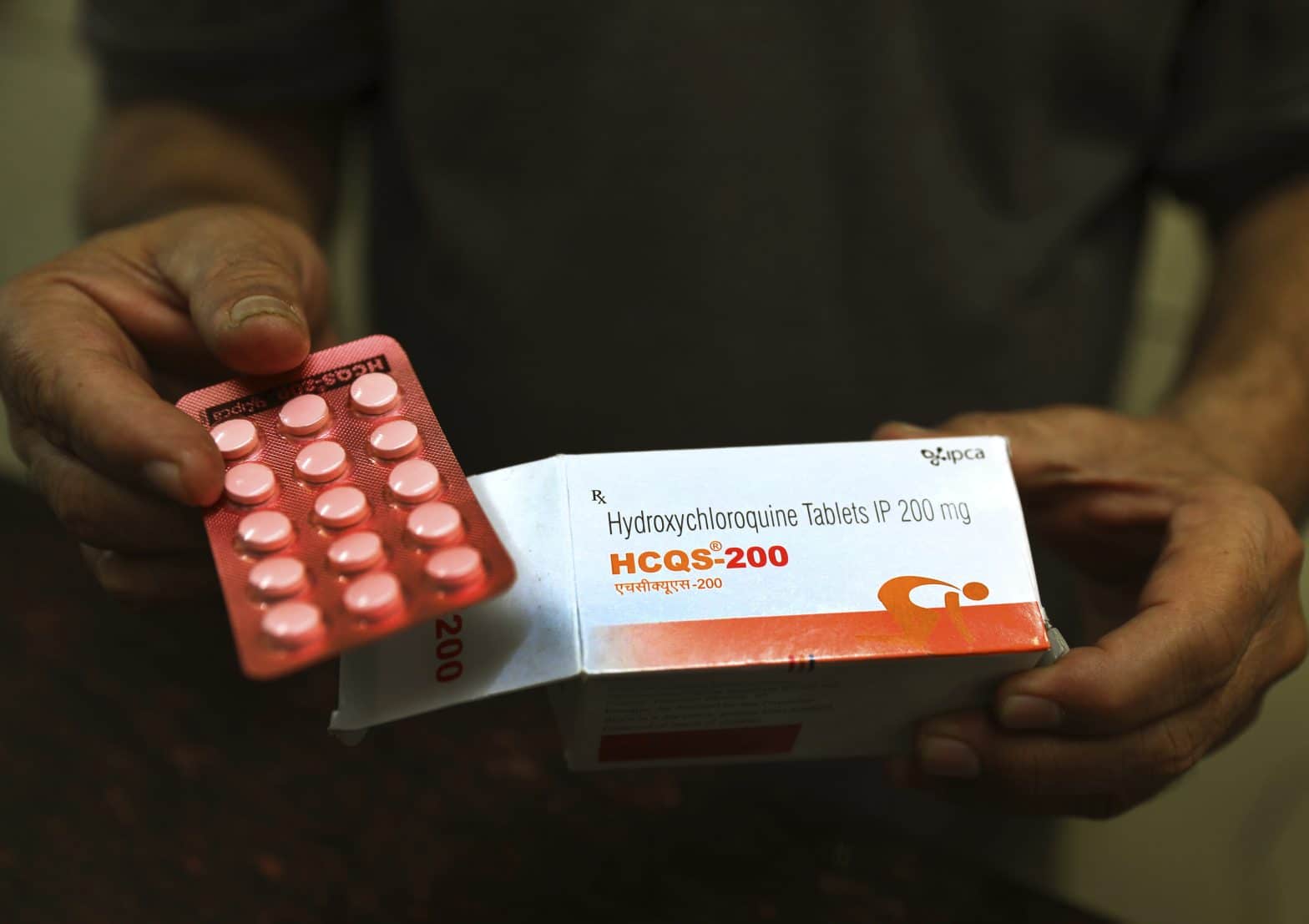
WASHINGTON – The Food and Drug Administration revoked its emergency authorization of hydroxychloroquine and chloroquine for treating COVID-19 as evidence mounts that the drugs don’t work and can cause serious side effects.
“We’ve made clear throughout the public health emergency that our actions will be guided by science and that our decisions may evolve as we learn more about the SARS-CoV-2 virus, review the latest data, and consider the balance of risks versus benefits of treatments for COVID-19,” said Dr. Anand Shah, FDA deputy commissioner for Medical and Scientific Affairs.
Dr. Patrizia Cavazzoni, acting director of the FDA’s Center for Drug Evaluation, said while additional clinical trials will continue to evaluate the potential benefit of these drugs in treating or preventing COVID-19, “we determined the emergency use authorization was no longer appropriate.”
Cavazzoni explained the decision to revoke the drugs emergency approval was taken following “a rigorous assessment” by scientists at the Center for Drug Evaluation and Research.
“We remain committed to using every tool at our disposal in collaboration with innovators and researchers to provide sick patients timely access to appropriate new therapies,” Cavazzoni said. “Our decisions will always be based on objective and rigorous evaluation of the scientific data. This will never change.”
President Donald Trump had aggressively pushed the drug beginning in the first weeks of the outbreak and stunned medical professionals when he revealed he took the drug preemptively against infection.
All this despite the fact no large studies have found the drugs safe or effective for preventing or treating COVID-19.
Last week, a National Institutes of Health panel of experts revised its recommendations to specifically recommend against the drug’s use except in formal studies
The decades-old drugs, also prescribed for lupus and rheumatoid arthritis, can cause heart rhythm problems, severely low blood pressure and muscle or nerve damage.
The FDA’s formal statement on the matter said, “in light of ongoing serious cardiac adverse events and other potential serious side effects, the known and potential benefits of chloroquine and hydroxychloroquine no longer outweigh the known and potential risks for the authorized use.”
The agency’s decision means that shipments of the drugs obtained by the federal government will no longer be distributed to state and local health authorities for use against the coronavirus.