FDA Panel Approves Pfizer Vaccine For Nationwide Distribution
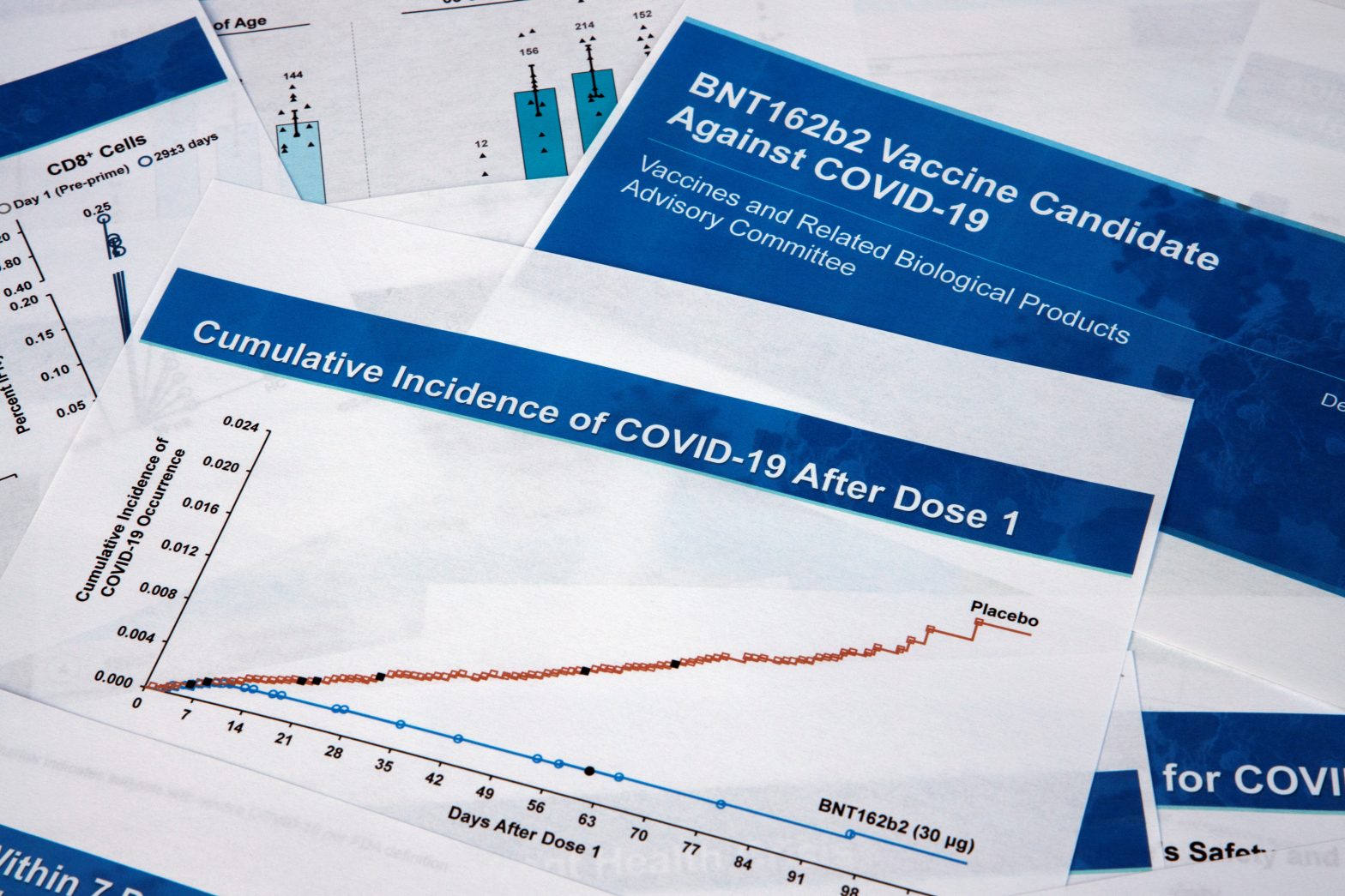
WASHINGTON — A federal panel of outside experts on Thursday endorsed a coronavirus vaccine from Pfizer and BioNTech, recommending that the Food and Drug Administration move forward with emergency authorization.
President-elect Joe Biden responded with a statement saying, “Today’s approval by the Food & Drug Administration of the Pfizer-BioNTech COVID-19 vaccine is a bright light in a needlessly dark time. We are grateful to the scientists and researchers who developed this vaccine. And, we are grateful to the scientists and public health experts who evaluated the safety and efficacy of this vaccine free from political influence.”
Hours earlier, cargo delivery company executives reassured a U.S. Senate committee that they are prepared for prompt and safe distribution of the COVID-19 vaccine.
They said they started preparing months earlier for what FedEx Express employees are calling “ship-a-thon.”
“We don’t expect anything we won’t be able to handle,” said Richard Smith, FedEx Express’ regional president of the Americas.
As Smith testified to the Senate Commerce, Science and Transportation Committee, a Food and Drug Administration advisory panel was meeting before voting later in the day to approve the Pfizer-BioNTech vaccine that shows 95% effectiveness in preventing COVID-19.
Nearly all the nationwide deliveries will be made by FedEx Express and United Parcel Service.
The urgency of the approval was underscored in comments by senators during the committee hearing amid reports of a record 3,140 COVID-19 American deaths a day earlier. Another record was set by the number of hospitalizations at about 106,000.
“We recognize that this is complex and critical work,” Smith said.
Approval of a second vaccine developed by Moderna, Inc. is expected within days.
Smith said the FedEx Express preparations included special training for the company’s cargo airline pilots, hiring an additional 70,000 employees and setting up equipment to produce the dry ice needed to keep the vaccines super cold.
Dry ice refers to frozen carbon dioxide, which is used as a cooling agent at sub-zero temperatures.
The Pfizer, Inc. vaccine must be stored at 94-degrees below zero Fahrenheit to preserve it before vaccination and the Moderna vaccine must be kept at 4 degrees below zero. In addition to dry ice, FedEx Express and UPS have put “ultra cold” freezers in their facilities.
Despite the logistical challenges, FedEx Express can deliver to every Zip Code in the United States “absolutely, positively overnight,” even in rural areas, Smith said.
Wesley Wheeler, president of Global Healthcare for UPS, said his company has been doing dry runs along with Pfizer and Moderna to ensure there would be no snags in deliveries.
“UPS has spent many weeks designing the supply routes,” Wheeler said.
The preparations included reserving air traffic lanes, building a computerized forecasting model to anticipate problems and installing machines at their Louisville, Ky., facility that can produce 40,000 pounds of dry ice daily.
The plans call for shipments transferred from airplanes to trucks for final delivery to be escorted by police. Many of the deliveries will go to pharmacies at Walgreens and CVS retail stores.
The vaccines will become UPS’s top priority, Wheeler said.
“It goes on the plane first, it comes off the plane first,” he said.
Several senators asked about the risk hackers could disrupt the distribution network through computer viruses.
Wheeler said UPS uses “data feeds” to track deliveries. Years of experience has taught their information technology security personnel to detect disruptions quickly, he said.
“Those data feeds are well protected,” Wheeler said. “We have firewalls.”
Sen. Tammy Duckworth, D-Ill., called the planned vaccine distributions “one of the most complex logistical challenges our country has ever faced.”
Sen. Jon Tester, D-Mont., seemed uncertain UPS and FedEx Express could make good on their pledge of prompt vaccine deliveries, particularly in rural areas like his home state of Montana.
“Quite frankly, if you get a blizzard that blows through, it’s going to screw things up,” Tester said.