American Powered Air-Purifying Respirators Set New Standard
At the onset of the COVID-19 pandemic, it became clear that the nation’s manufacturing capabilities were ill-prepared to respond to the urgent needs of the medical community.
As the pandemic’s scope progressed, so did China’s chokehold on supplying countries with personal protective equipment and other front-line medical hardware such as masks, testing kits and respirators. One company, however, Brooklyn-based American PAPR, has taken the initiative to fulfill the demand for PPE and powered air-purifying respirators by drawing from domestic resources, supply chains, manufacturing and labor.
Giles Kyser, a retired United States Marine Corps colonel and CEO of American PAPR, said that while air respirators like the ones his company produces were commonly used prior to the pandemic in a variety of fields, the threat faced by front-line medical workers in combating the spread of the virus became their new focus.
“There did not exist the density of powered air-purifying respirators or PPE at large to protect the large numbers of people that found themselves exposed to the threat,” Kyser said. “Back during that time, people were going nuts trying to get their hands on the N95 masks and gloves and face shields. People were doing all manner of things trying to protect themselves because there was a lot of uncertainty with it.”
From inception to implementation, American PAPR completed its product certification process in just seven weeks. Kyser said these procedures usually take up to a couple of years to complete, highlighting the level of commitment his company has to its mission of adequately addressing current and future pandemic threats.
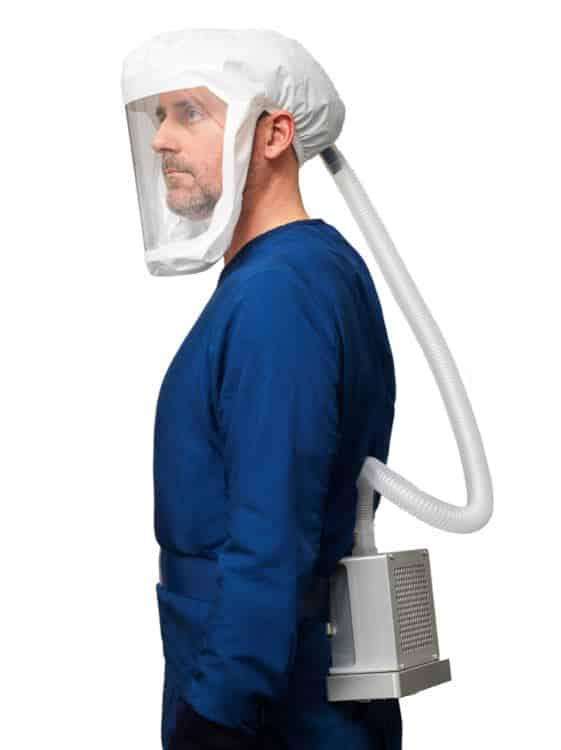
Powered air-purifying respirators represent the most effective way to protect at-risk medical workers by virtue of the technology they encompass and the level of protection the devices provide, Kyser said. In addition, they provide an unmatched level of comfort while still allowing for nonverbal communication between medical personnel and patients.
American PAPR’s FELIX™100 is the first respirator approved under new National Institute for Occupational Safety and Health standards for health care workers, and the product’s high-efficiency particulate air filters are certified to have 99.97% effectiveness at capturing particulates of 0.3 micrometers or larger.
“When you have an uncertain threat environment, when you have a pandemic which is spreading at the rate at which it was, you can see the sense of urgency that existed inside of those hospitals,” Kyser said. “And that’s where American PAPR saw its opportunity to do good and do well, simultaneously.”
Not only would the company be doing good for medical personnel in general, Kyser said this presented an opportunity to establish a precedent for the return of American reliance on domestic manufacturing and supply chain independence. Instead of depending on China’s supply of PPE, the company could create a template for supporting American jobs going forward whenever instances of deep economic despair arise.
Kyser’s sentiments reflect a reality not lost on the Biden-Harris Administration. In February, President Joe Biden formally ordered a government review of potential vulnerabilities in U.S. supply chains for crucial commodities, intending to shore up weaknesses that led to shortages of PPE in the early stages of the pandemic last year.
Further, Kyser said these products will see usage post-pandemic as well. They can be used not only by doctors and nurses in hospitals but also for respiratory protection in law enforcement, military and industrial settings.
“This is where we need federal and state government to come in on the side of doing the right thing,” Kyser said. “Not only for the doctors and nurses, but doing the right thing for the nation by saying, ‘Look, we’re going to stop spending taxpayer money on state and federal acquisitions that don’t use American companies.’ We like being part of that solution.”























